Shatter Expectations
Clinically Proven with Foundational IOP Control
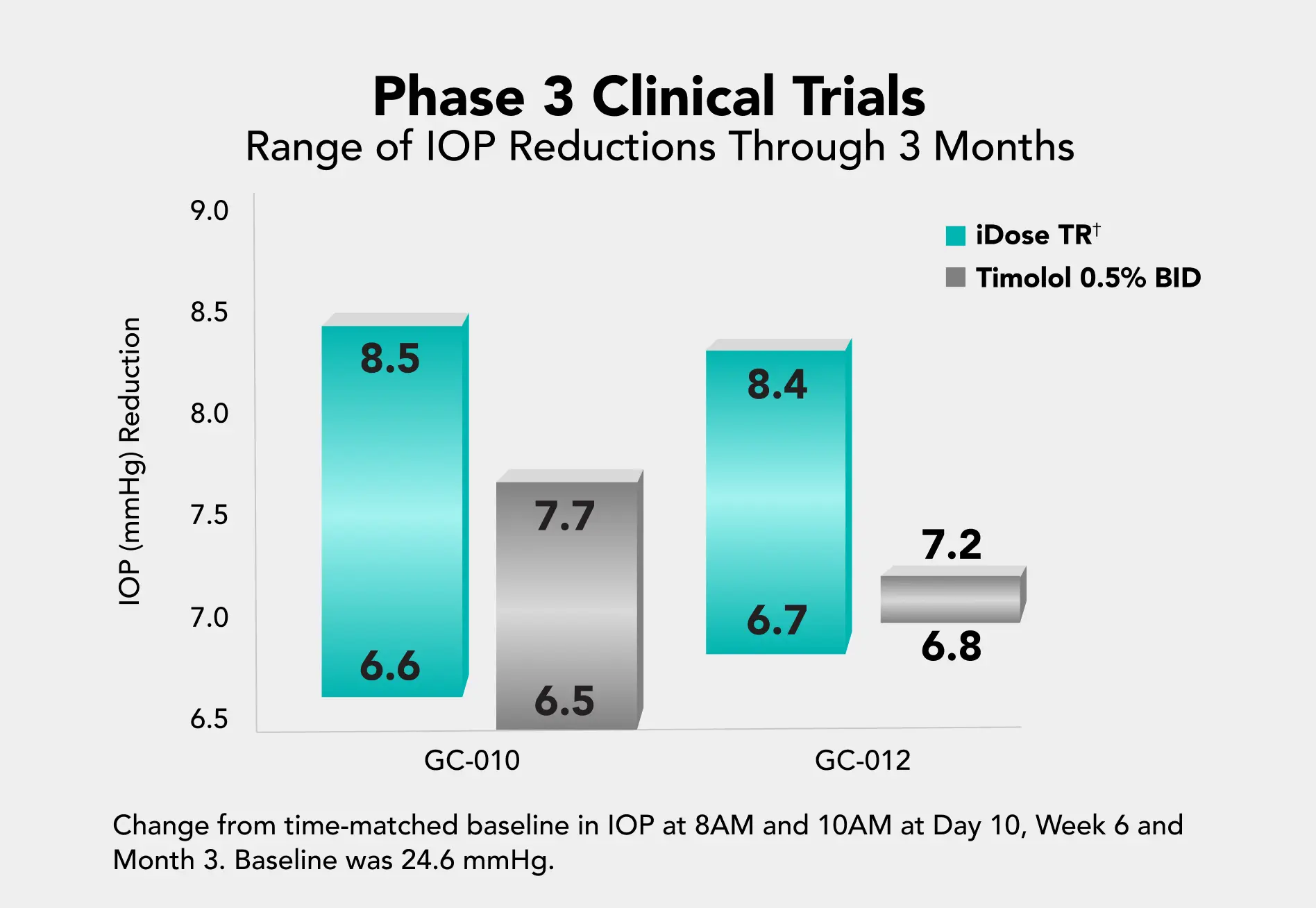
In two pivotal trials, iDose TR achieved the pre-specified primary efficacy endpoint (non-inferiority to topical timolol through 3 months).1
Non-inferiority of iDose TR to timolol was established if the upper limit of the two-sided 95% confidence interval for the difference in the mean of change from baseline in IOP was <1.5 mmHg at each of the six post-baseline timepoints and was < 1 mmHg at half or more of the six post-baseline timepoints.
PHASE 3 STUDY DESIGN
To evaluate iDose TR efficacy and safety in patients with ocular hypertension and open-angle glaucoma. Two identically-designed Phase 3, parallel-group, double-masked, randomized, prospective, sham-controlled trials compared iDose TR to topical timolol (0.5%) BID. N=1150; 89 trial sites1*
PRIMARY ENDPOINT
Change from baseline in diurnal IOP (vs timolol) through 3 months postoperative.1**
Enduring Control at 12 Months†
- 81%of iDose TR subjects were completely free of IOP-lowering topical medications
(23% were originally on 2+ medications pre-trial)1ABOUT
iDOSE TRLearn More iDOSE TR
SAFETYLearn More Ready to hear more?
Contact a representative to request more info.
1. iDose TR Phase 3 Clinical Trials, data on file, Glaukos Corporation.
*Inclusion Criteria: Patients 18 years or older; diagnosed with open-angle glaucoma (POAG, PXG or PG) or ocular hypertension; C/D ratio ≤ 0.8; zero to three preoperative ocular hypotensive medications; BSCVA of 20/80 or better.
** Change from time-matched baseline in IOP at 8AM and 10AM at Day 10, Week 6 and Month 3.
† iDose TR clinical results shown represent the approved and commercially marketed version; identified as the “slow-eluting implant arm” in the pivotal trials.